“The following are the latest risk figures for PML as a result of being treated with natalizumab. Please note that the embedded slideshow is for health professionals only; if you are not a health professional Biogen-Idec don’t want you to see this presentation. If you are a MSer you should be reading my previous post that has been designed for MSers. There is a change in that Biogen-Idec will only be providing these figures from now on every quarter.
Headline information
“As of the 3rd December 2014 there have been 517 cases of natalizumab-associated PML; an increase of 22 cases over the previous 3 months. Over 132,600 MSers have been exposed to natalizumab. The following graph demonstrates the number of new PML cases per month seems to be relatively stable, despite a gradual and linear increase in number of exposed MSers. Clearly the ratio is decreasing which indicates that the PML de-risking programme is working; in other words less MSers at risk of PML are staying on the natalizumab. Herein lies the problem; this means that the proportion of JCV-ve MSers on natalizumab is increasing. However the total number of MSers on treatment is used in the denominator to calculate the risk of getting PML. If this denominator is changing by including an increasing proportion of MSers who are not at risk of getting PML it will give a falsely low risk of PML. What we need are monthly updates of the PML risk, by excluding all JCV-ve MSers from the analysis. Unfortunately, Biogen-Idec are unable to access this information, despite them providing the JCV antibody assay free. Why? They don’t have consent from the MSers who are being tested for JCV to use their data in this way.”
“The following ratios are my attempt to explain why I think we are under-estimating the PML risk. At present Biogen-Idec is calculating the PML risk using the top equation. What I would like to see are PML risks calculated using the lower equation.”
“The overall mortality associated with PML was 23% in December; in other words 119 MSers have died as result of PML. Please note that the majority of the PML survivors have a poor functional outcome. You need to keep these figures in context of over 132,600 MSers been treated with natalizumab worldwide with over 381,000 years of natalizumab exposure.”
“Since NHS England gave us permission to switch high-risk natalizumab patients to fingolimod, we are continuing to de-risk our natalizumab-treated population. We are hoping by doing this to prevent anyone at our centre from getting PML. Despite this some MSers are not prepared to stop natalizumab, simply because they are doing so well on the drug.”
“The following is the most important headline data slide for MSers regarding risks based on the three identified PML risk factors:
- JCV serostatus
- Duration of treatment
- Previous exposure to immunosuppression
In addition to this is appears that titres or levels of anti-JCV antibodies also play a role in risk (see below) and this needs to be incorporated into future risk models.”
“We have developed a simple infographic to help you integrate all this information. You can download and print this infographic for your own information.”
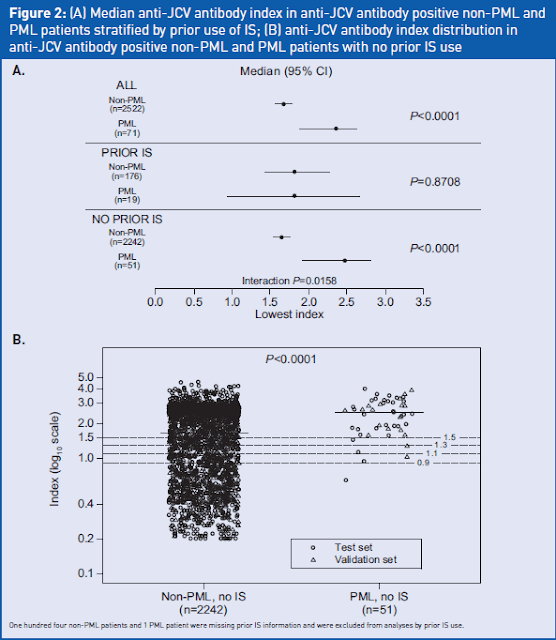
Objectives: In MSers treated with natalizumab, the presence of anti-JCV antibodies (JCV Ab+), prior use of immunosuppressants (IS), and increased duration of natalizumab treatment, especially greater than 2 years, are known risk factors for progressive multifocal leukoencephalopathy (PML). With polyomaviruses, higher levels of antibodies have been correlated with increased viral burden and increased disease risk. It is not known whether JCV Ab levels correlate with PML risk in natalizumab-treated MSers. The objective of this analysis is to examine the association between JCV Ab index (JCV antibody level as measured using the STRATIFY JCV DX Select assay) and PML risk in natalizumab-treated MSers.
Methods: Analyses involved JCV Ab index data from JCV Ab+ MSers enrolled in clinical studies or clinical practice. A cross-sectional analysis of JCV Ab index data from MSers without PML was first performed to assess potential relationships between JCV Ab index and known risk factors (natalizumab treatment duration <=24 vs >24 monthly infusions and prior IS use). P values were calculated using a Wilcoxon rank sum test. The association between JCV Ab index and PML was then assessed using all available longitudinal data. Odds ratios (ORs) were estimated from generalised estimating equations with a logit link. The predicted probabilities were then used to update the current PML risk estimates for JCV Ab+ MSers with high/low Ab index by applying Bayes theorem.
Results: JCV Ab index data were available from 71 natalizumab-treated PML MSers at least 6 months prior to PML diagnosis and from 2522 non-PML JCV Ab+ MSers. JCV Ab index was not found to be associated with number of natalizumab infusions (P=0.39) nor prior IS use (P=0.43), but was significantly associated with PML risk (P<0.001). Estimated ORs were at least 4 for high versus low JCV Ab index in JCV Ab+ MSers. Updated PML risk estimates and longitudinal stability of JCV Ab index will be presented.
Conclusion: Risk of PML in JCV Ab negative natalizumab-treated MSers is very low (0.07 per 1000). In JCV Ab+ MSers who have low JCV Ab index, the risk of PML is several-fold lower than the risk currently attributed to all JCV Ab+ MSers. Utilisation of JCV Ab index allows for further clinically meaningful stratification of PML risk in JCV Ab+ natalizumab-treated MSers.






Yes, PML risk is low. But what about all the non-PML adverse events that result from Tysabri use? These go unreported despite often being devastating. I had a severe rebound event that attacked my brain stem during the washout period that was recommend to prevent PML when a blood test showed I'd switched to JC positive after two years on Tysabri. My neurologist followed the recommend protocol of protective steroid infusions during the wash out. It didn't matter and I ended up crippled. What's maddening is that this was considered a "success" because I avoided PML. How many similar Tysabri patients suffered a similar miserable success? We don't know because the adverse event data other than PML isn't reported.
Given the availability of Alemtuzumab, why would a neuro suggest Tysabri as a treatment option? I can't see a reason given that Alemtuzumab has higher efficacy, is cheaper in the long run, is more convenient (usually five days or infusions and three infusions a year later) and safer (see above for PML risk).
"AnonymousFriday, January 30, 2015 7:02:00 pmGiven the availability of Alemtuzumab, why would a neuro suggest Tysabri as a treatment option?"I'd also like to know too, please.
Too risky. Not interested. What a vile treatment option.
Why don't Idec want MSers to read the slideshow? What about health professionals who have MS – read half maybe.
I am more concered with the rebound effect then the pml risk !
'AnonymousFriday, January 30, 2015 7:41:00 pm"AnonymousFriday, January 30, 2015 7:02:00 pmGiven the availability of Alemtuzumab, why would a neuro suggest Tysabri as a treatment option?"I'd also like to know too, please.'Me too but it seems this request is met with a resounding silence.
im not a neuro but maybe patient preference eg. jc virus negative person
Re: "Given the availability of Alemtuzumab, why would a neuro suggest Tysabri as a treatment option?"I have posted on this topic before it is not easy and is very complex. I think MSers need the choice; not everyone wants an induction therapy. Induction therapies are irreversible and come with risks. We also don't know what the long-term consequences of alemtuzumab therapy; some MSers therefore prefer being treated with a drug that is reversible. Please read the my previous post: http://multiple-sclerosis-research.blogspot.com/2014/08/clinicspeak-natalizumab-or-alemtuzumab.html
Have you guy's seen this paper on PML risk? http://vixra.org/abs/1504.0148
Very interesting. I wonder what Biogen-Idec will have to say about these new figures. Any idea who Julian Borchardt is? I can't find any link to this name and MS on Google.
Even I would like to know!
yes ive seen it
This is a very interesting paper, thanks for sharing. The bombshell in this paper is the nature of the error in Biogen's PML estimates. The error wasn't due to a subtle source of bias, e.g. the derisking of the Tysabri-taking population, which Prof G has previously talked about. This was high school algebra. Biogen was calculating the probability of getting PML during the first 4 years of Tysabri treatment. To do this, they looked at the number of people who got PML during the first 4 years of treatment, and divided this by the total number of people who took the drug for 4 years. So far, so good. They decided, though, that they would count someone who took Tysabri for 2 years and 1 month as someone who had taken the drug for 4 years. The chance of this person getting PML is obviously much lower than the chance of someone who had really taken the drug for 4 years getting PML. So in their denominator, they had a lot of people who had taken the drug for much less than 4 years, counting as people who had safely taken the drug for 4 full years. Not surprisingly, this resulted in a substantial understatement of PML risk.To give an analogy, suppose that we wanted to estimate the chance of living to 120. If we followed Biogen's approach here, then every living person would count as someone who had lived to 120 — because they hadn't yet died before that age. The fact that this appeared in NEJM is unsettling.
Julian Borchardt was a German socialist journalist activist (1868-1932) and the question is it this written by a real person or a pseudonym as the email address from an email providerAbstractWe show that the quarterly updates about the risk of PML during natalizumab therapy, while in principle helpful, underestimate the real incidences systematically and signi cantly. Calculating the PML incidences using an appropriate method and on realistic assumptions, we obtain estimates that are up to 80% higher. In fact, with the recent paper [Plavina et al 2014], our approximate incidences are up to ten times as high. The present article describes the shortcomings of the methods used in [Bloomgren et al 2012] and by Plavina et al for computing incidences, anddemonstrates how to properly estimate the true (prospective) risk of developing PML during natalizumab treatment. One application is that the newest data concerning the advances in risk mitigation through the extension of dosing intervals, although characterised as not quite statistically signi cant, are in fact signifi cant. Lastly, we discuss why the established risk-strati cation algorithms, even on assessing the PML incidences correctly, are no longer state-of-the-art; in the light of all the progress that has been made so far, already today it is possible to reliably identify over 95% of patients in whom (a personalised regimen of) natalizumab should be very safe.I am aware of this paper and have been interested to see if and how Biogen would respond, So far nothing that I am aware. ProfG and the blog is cited a lot and maybe he wants to comment or has heard from Biogen as they no doubt should have read this by now.But the question is who has reviewed this paper, it is 45 pages long are the figures reliable, Are the methods reliable. We can all read the words but are they based on a solid foundation…..Come on Biogen break your silence and post some comments
You may be interested in this paperReassessing the risk of natalizumab-associated PML.Berger JR, Fox RJ.J Neurovirol. 2016 Aug;22(4):533-5."The current numbers indicate substantially higher risks for PML in 2015 than in 2012. Our calculations suggest that an individual having all three risk factors has an approximately 1 in 44 chance of developing PML"
Anonymous 4:03 here. The Borchardt paper has now been published in MSARD: http://www.ncbi.nlm.nih.gov/pubmed/27456891Though it was obviously correct at the time of its initial dissemination, to anyone who bothered to read it carefully, it's good that it has received this stamp of approval from a major journal.
Has the decrease continued into 2015? Still nothing from Biogen on the Borchardt paper??