Background: BG-12 (dimethyl fumarate) is an experimental oral treatment for relapsing-remitting multiple sclerosis (RRMS) that may have dual anti-inflammatory and neuroprotective effects via the Nrf2 pathway. In a Phase 2b trial, BG-12 reduced inflammatory activity in patients with RRMS.
Objective: To report the effects of BG-12 on clinical efficacy endpoints in the Phase 3 DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS) study.
Methods: DEFINE was a randomised, double-blind, placebo-controlled, multicentre clinical trial that evaluated the efficacy and safety of BG-12 over 2 years in patients with RRMS. Patients aged 18-55 years with McDonald criteria diagnosis of RRMS and an Expanded Disability Status Scale (EDSS) score of 0.0-5.0 (inclusive) were randomly assigned on a 1:1:1 basis to placebo, BG-12 240 mg PO twice daily (BID), or BG-12 240 mg three times daily (TID). All subjects underwent clinical assessments at screening, baseline, and every 4 weeks for up to 2 years. The primary endpoint was proportion of patients relapsing at 2 years, with relapses confirmed by an independent neurology evaluation committee to ensure consistent and accurate reporting across sites. Secondary clinical efficacy endpoints at 2 years were annualised relapse rate (ARR) and disability progression using EDSS. Efficacy analyses were conducted on the intention-to-treat population.
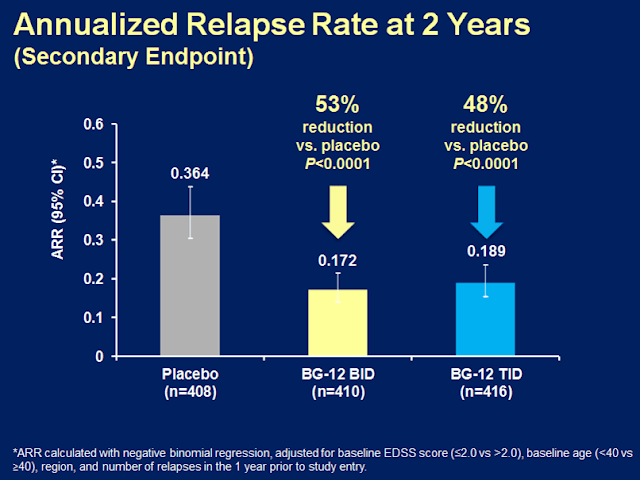
Results: A total of 1234 patients were dosed with placebo (n=408), BG-12 BID (n=410), or BG-12 TID (n=416), with similar demographic and clinical characteristics across treatment groups. All primary and secondary endpoints of the study were met. BG-12 BID and TID reduced the risk of relapse by 49% and 50%, respectively, compared with placebo (P<0.0001) at 2 years. ARR was 0.36 with placebo, and 0.17 and 0.19 with BG-12 BID and TID, corresponding to reductions of 53% and 48% for BG-12 BID and TID (P<0.001). The risk of confirmed 12-week disability progression was reduced by 38% with BG-12 BID (P<0.01) and by approximately 34% with BG-12 TID (P<0.05). The overall incidence of adverse and serious adverse events was similar among the placebo group and both BG-12 treatment groups.
Conclusions: The results from this large Phase 3 study support the potential of BG-12 as an effective oral treatment for patients with RRMS that has a novel mechanism of action.
“Although this is old news these results were one of the highlights of the meeting. BG12 is based on a drug that has been used for several years in psoriasis with a good side effect profile. In other words it seems to be safer than other drugs with this level of efficacy. Another positive attribute is that BG12 may have neuroprotective effects based on its mechanism of action; this makes it a great candidate for testing in progressive MS.”
CoI: Multiple

Hurray for everone with RRMSS!How about you write up something positive for PPMS sometime?
Any idea on when BG 12 may be available in the United States? Would this be a suitable add on therapy with one of the current injectables?
Why not instead of injectables? Same level of efficacy and good safety profile, and much preferable method of administration.
@ Anon,The reason I asked as on an add on was because there may be added benefits from combined usage that would be superior to either BG 12 or interferon/copaxone alone, but I am sure insurance companies would be thrilled to cover both at the same time. Besides method of administration and safety, it appears that BG 12 may be a bit more efficacious than the injectables based on disability reduction and relapse rates but that only way to know for sure would be a head to head trial.
Re: "How about you write up something positive for PPMS sometime?"Unfortunately, there is nothing to report at the moment. We will need to wait 3 to 4 years for the Fingolimod PPMS trial to run its course. The results will probably be presented at the 2014 ECTRIMS.
Re: "Any idea on when BG 12 may be available in the United States? Would this be a suitable add on therapy with one of the current injectables?"The company will have to submit the data for registration; ~ 6 months time. The FDA and EMA will then have to rule on the drug; without accelerated approval this will take 12 months. So I suspect the drug will only be available in the US in mid 2013. It will not be an add-on, but used instead of the injectables. Trials testing it as an add-on will need to be done.
Re: "Why not instead of injectables? Same level of efficacy and good safety profile, and much preferable method of administration."I agree!
Perhaps if Biogen make a shedload of money out of BG12 taking over Rebif's pitch, they will resurrect their research into Baminercept.
Re: "Baminercept"I agree; why not start a lobby. A Facebook page and Twitter account; "Progressive MS'ers for Baminercept". They may take note. If the CCSVI lobby can get the Canadian and Italian governments to take note why not Progressive MS'ers for Baminercept and Biogen-Idec?This is one lobby group I would gladly join!